Helium-3 isn’t just a fascinating scientific curiosity. It’s a rare isotope with the potential to transform how we power our world. As interest in space mining ramps up, the conversation around Helium-3 has taken center stage, especially when it comes to its presence on the Moon and what it might mean for future energy solutions.
So, let’s break down why Helium-3 is attracting attention, how it could power future fusion power plants, why we find it on the Moon and not Earth, and who is racing to extract it.
What Is Helium-3?
First things first, let’s start with the basics and explain what Helium-3 is. Helium-3 (3He) is a light, stable isotope of helium with two protons and one neutron. Unlike the more common helium-4, which has two neutrons, helium-3 is non-radioactive and does not decay over time. It’s extremely rare on Earth, occurring only in trace amounts as a result of the decay of tritium or being released from the Earth’s crust.
Because of its unique nuclear properties, helium-3 has attracted attention as a potential fuel for fusion reactions. When used in fusion reactions, helium-3 produces energy without the dangerous neutron flux and long-lived radioactive waste typically associated with nuclear power, making it an especially attractive option for clean fusion energy.
Helium-3 is also used in various scientific applications, which we will cover a bit later in more detail. But with Earth’s helium-3 stockpile limited and costly to replenish, scientists have looked to the Moon as a potentially more sustainable source compared to terrestrial supplies.
Why Is Helium-3 on the Moon and Not on Earth?
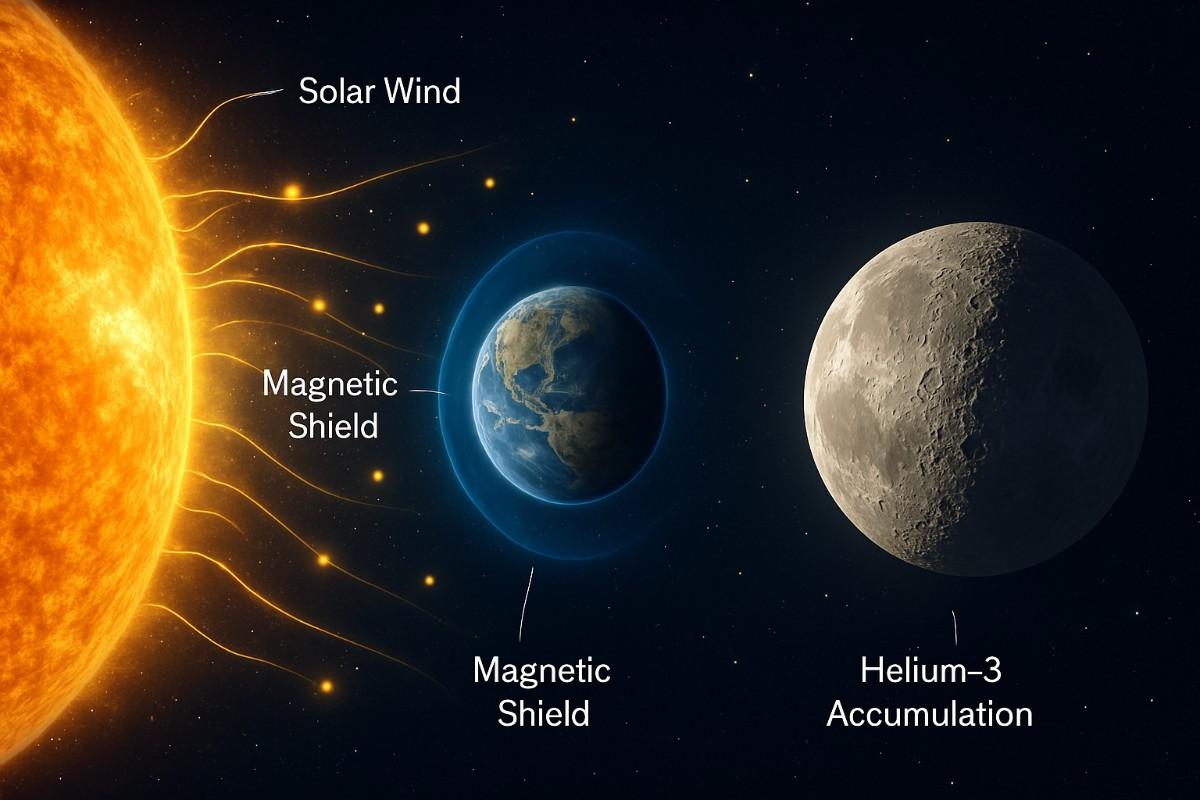
Now, we’ve said that helium-3 is an isotope of helium that is extremely rare on Earth but relatively abundant on the Moon. Why the difference?
The answer lies in the solar wind. This constant stream of charged particles from the Sun, including helium-3 produced in the Sun’s core, bombards the lunar surface, which has no atmosphere or magnetic field to deflect it. Over billions of years, particles like helium-3 embedded themselves into the Moon’s topsoil, or regolith. On Earth, our protective atmosphere and magnetic field block most of the solar wind, and geological activity buries anything that does land.
Studies estimate there are substantial quantities of helium-3 on the Moon, potentially over a million metric tons embedded in the upper few meters of soil. While its natural abundance is still very low (about 20 parts per billion), the abundance of helium-3 on the Moon is several orders of magnitude higher than on Earth.
Why Is Helium-3 So Valuable?

Helium-3’s value comes from what it could do in the future: power nuclear fusion reactors that generate clean, safe, and efficient energy. Traditional nuclear power plants rely on nuclear fission, splitting heavy atoms like uranium, which produces long-lived radioactive material and hazardous waste. Fusion, by contrast, combines light atoms like hydrogen to form helium, releasing vast amounts of energy with little waste.
Most current fusion research focuses on D-T fusion – the reaction between deuterium and tritium. However, this reaction produces a lot of energetic neutrons, which damage reactor walls and make them radioactive over time. It also uses tritium, which is hard to handle due to the radioactive decay of tritium.
Here’s where helium-3 comes in. When combined with deuterium, helium-3 can fuel an aneutronic fusion reaction. That means minimal neutron flux, less radiation, and less wear on equipment. In theory, fusion reactions using helium-3 could power fusion energy plants with none of the long-term nuclear waste problems of today.
Scientists like Gerald Kulcinski, a leading expert in plasma physics and fusion research, have long championed helium-3 as the ideal fuel for clean fusion power. Helium-3 also has practical value today. It’s used in quantum computers as a cryogenic coolant, particularly in dilution refrigerators, which are essential for maintaining the ultra-low temperatures required to stabilize quantum bits (qubits).
In national security applications, helium-3 is a key component in neutron detectors used for scanning cargo and detecting nuclear material, especially at ports and border crossings. These detectors rely on helium-3’s sensitivity to neutrons, allowing them to identify even small amounts of radioactive material.
In the medical field, helium-3 can be used in hyperpolarized gas MRI, a specialized imaging technique that provides detailed views of lung function by using inhaled helium-3 gas as a contrast agent. But Earth’s helium-3 stockpile is tiny. We can’t produce it in substantial quantities without waiting for the decay of tritium, which is slow and expensive.
What Would Helium-3 Mining Look Like on the Moon?

Now, let’s get practical. What does mining helium-3 from the Moon involve? Since helium-3 is embedded in lunar regolith, miners would need to:
- Scoop and collect soil from the upper few meters of the lunar surface using robotic excavation tools designed to withstand lunar dust and temperature extremes.
- Heat the regolith to around 700°C, usually through solar or nuclear-powered furnaces, to release trapped gases that include both helium-3 and helium-4.
- Separate and extract the helium-3 through advanced cryogenic and chemical processes that can isolate the helium-3 atom from the other volatiles present.
Because of its low concentration, you’d need to process millions of tons of soil to harvest a few hundred kilograms. That’s not easy – it requires new technology and robotics, substantial energy supplies, and highly efficient gas-separation infrastructure. Specialized harvesting rovers would likely work in tandem with stationary heating and extraction units to optimize throughput.
To put the scale in perspective, the amount of lunar soil that would need to be processed is comparable to what some of the largest mines on Earth handle each year. While such operations are achievable here, replicating that scale on the Moon presents a significant logistical and engineering challenge.
Operations would likely need to be semi-autonomous, using AI-guided machines to handle the harsh environment and delicate extraction processes with minimal human intervention. Maintenance systems would need to be modular and remotely serviceable.
Transportation systems would need to carry the extracted helium-3 from the Moon to Earth, potentially using existing infrastructure like the Kennedy Space Center for delivery and distribution. While mining will require significant infrastructure and energy investment, the transport itself poses less of a challenge — only a few tons of helium-3 would be needed annually, making return missions relatively manageable in terms of mass.
Who Is Planning to Mine Lunar Helium-3?
Several companies and agencies are making bold moves toward Helium-3 extraction. Here are the most important ones:
- Interlune is a Seattle-based startup founded by former Blue Origin engineers and Apollo astronaut Harrison Schmitt. They’ve raised over $15 million to launch a mission by 2028, focusing directly on lunar helium-3 harvesting.
- ispace, a Japanese space company, has partnered with Magna Petra, aiming to extract and eventually transport helium-3 to Earth. Their early mission tests focus on lander tech and small-scale regolith sampling.
- NASA and international agencies (including China, India, and Russia) are eyeing lunar resources, including helium-3, for long-term infrastructure. While not all are focused solely on helium-3, it often features in their long-range goals.
The commercial interest is clear. With rising demand for helium in quantum computers and the potential of fusion energy, the race is on to unlock helium-3’s value.
Can Helium-3 Deliver?
It’s important to temper the excitement with realism. While Helium-3 sounds like a miracle fuel, we still haven’t built working nuclear fusion reactors that rely on it. Most fusion experiments today use D-T fuel because it’s easier to ignite.
However, scientists and engineers are pushing boundaries. Research into aneutronic fusion, better confinement methods, and new materials is making helium-3 more plausible as a fuel of the future. If we succeed, it could power a new generation of safe, clean nuclear power plants with minimal radioactive material.
But, until then, helium-3 remains a symbol of what’s possible in the age of space resource utilization. From the solar wind to the Moon’s lunar surface, nature has left us with a riddle worth solving, and the question is: who will get there first, and can they make it work?