Producing fuel in space is no longer just a concept from science fiction. It’s becoming a crucial part of how we plan future missions, from setting up a permanent moon base to enabling long-term space travel across our solar system. Instead of carrying every drop of fuel from Earth – an incredibly expensive and weighty task – we’re learning how to make it directly on the Moon, Mars, and even in deep space.
Let’s break down the types of rocket fuels used today, explore what we can actually produce beyond Earth, and how this all connects to space mining, resource utilization, and the future of refueling in space!
Rocket Fuels 101: What Powers a Rocket?
Before we explore how to make rocket fuel in space, it’s good to understand what fuels rockets in the first place. Most rockets today rely on either liquid, solid, or hybrid fuel systems, each with its own set of advantages and tradeoffs.
- Liquid rocket fuels, such as liquid hydrogen and liquid oxygen, are widely used for their efficiency and power. These are stored as cryogenic propellants, kept at extremely low temperatures to stay in liquid form. When ignited in a combustion chamber, they produce massive amounts of thrust. This fuel type powers vehicles like NASA’s Space Launch System. Notably, these fuels offer one of the highest specific impulses (a measure of efficiency), which makes them well-suited for large payloads and long-duration missions. However, maintaining them at cryogenic temperatures is technologically demanding, especially for long-term storage in space.
- Solid rocket fuels, which combine both fuel and oxidizer into a single block, are often composed of powdered metals and a polymer binder. These are common in solid rocket boosters and solid rockets used during liftoff, such as those on the former Space Shuttle. Once ignited, the burn can’t be stopped or throttled, making them simple and powerful, but not flexible. Their shelf-stability and rugged design make them ideal for launch vehicles and emergency escape systems.
- Hybrid fuels combine a solid fuel with a liquid oxidizer, offering greater control than solid rockets and a simpler design than full liquid systems. They’re still relatively rare, but test systems like Virgin Galactic’s early SpaceShipTwo flights have used them.
- For deep-space missions, thermal rockets and electric propulsion systems come into play. These use solar energy or nuclear power to heat and accelerate a gas, often xenon or hydrogen, through an engine. They don’t provide much thrust but are incredibly efficient, making them ideal for fuel-efficient travel over long distances in space. However, because of their low thrust, they aren’t suitable for lifting off from Earth’s surface — they’re best used once a spacecraft is already in orbit.
It’s essential to understand these types of fuel because it will help explain why producing different fuel components in space is so important and why it’s a major focus of modern space exploration.
Making Fuel Beyond Earth
To produce rocket propellant beyond Earth, we use a concept called In-Situ Resource Utilization (ISRU). This involves using local resources – such as water, atmospheric gases, and minerals – to generate essential supplies like fuel, breathable oxygen, and water. By reducing our dependence on Earth-based resupply missions, ISRU makes it possible to extend our reach and duration in space. Let’s take a closer look at what this looks like on different celestial bodies.
The Moon: Oxygen, Water, and Regolith
The Moon may seem barren, but its surface is packed with potential. One of the most promising resources is water ice, identified in permanently shadowed craters near the lunar south pole. Although NASA’s VIPER mission, which aimed to map and analyze these deposits, was canceled in 2024 due to budgetary constraints, the search for lunar water ice continues through other planned missions and international collaborations.
Once harvested, the ice can be melted and electrolyzed to produce oxygen and hydrogen from water. Together, these gases form a powerful combination for liquid fuel rockets, especially when cooled into cryogenic propellants.
In addition to ice, the lunar regolith – a fine, dusty soil – contains oxygen bonded within its minerals. NASA and other agencies are developing techniques to extract oxygen through molten regolith electrolysis, which could contribute both to fuel production and a steady supply of breathable oxygen for astronauts.
Even more novel is the idea of using the regolith in thermite reactions. A University of Waterloo study demonstrated that mixing lunar soil with aluminum (potentially sourced from old satellites or lander parts) can trigger a high-temperature reaction, releasing energy or freeing oxygen – an approach that could be useful for thermal rockets or auxiliary power.
These technologies not only enable solid propellant rockets and liquid-fueled systems to be refueled locally, but they also bring us closer to establishing a self-sustaining lunar base.
Mars: CO2, Ice, and Fuel from the Air
Mars offers a unique set of ingredients for in-situ fuel production. Its thin atmosphere is made up of more than 95% carbon dioxide, making it a natural candidate for CO₂-based fuel systems.
NASA’s MOXIE experiment, carried out on the Perseverance rover, successfully used high-temperature electrolysis to convert Martian air into liquid oxygen. A larger version of this system could support crewed return missions by generating the oxidizer needed for liftoff.
Of course, ice deposits on Mars play a crucial role when it comes to resources. Mars contains subsurface water ice, which can be melted and split into hydrogen and oxygen. The hydrogen can then be combined with CO₂ using the Sabatier reaction, producing methane – a perfect fuel for liquid fuel rockets like SpaceX’s methane-powered Starship. This combination offers a closed-loop system where water and air become fuel and life support materials.
Further down the road, experiments in artificial photosynthesis conducted by Chinese astronauts aboard the Tiangong Space Station could be adapted for Mars.
Together, these options could support not just rocket launches from Mars but also fuel local power systems, mobility vehicles, and emergency oxygen reserves.
Asteroids: Still Theoretical, But Valuable
Although no asteroid has been mined yet, they hold promise as future space refueling stations. Certain types, like carbonaceous asteroids (C-type), are rich in water vapor, volatile compounds, and organic materials. These asteroid resources could be captured and processed through electrolysis to generate hydrogen and oxygen, ideal for refueling liquid fuel rockets or supporting long-haul space missions.
Metal-rich asteroids also contain elements like magnesium and aluminum, which could be repurposed for solid propellant rockets or utilized in thermite reactions to release energy in environments where solar or nuclear power is limited.
While the necessary technology is still in development, asteroid mining remains one of the most exciting and potentially game-changing frontiers in space exploration. As robotic mining missions become more viable, asteroids could evolve from targets of study into essential stops on interplanetary routes.
How Do We Turn Raw Resources Into Rocket Fuel?
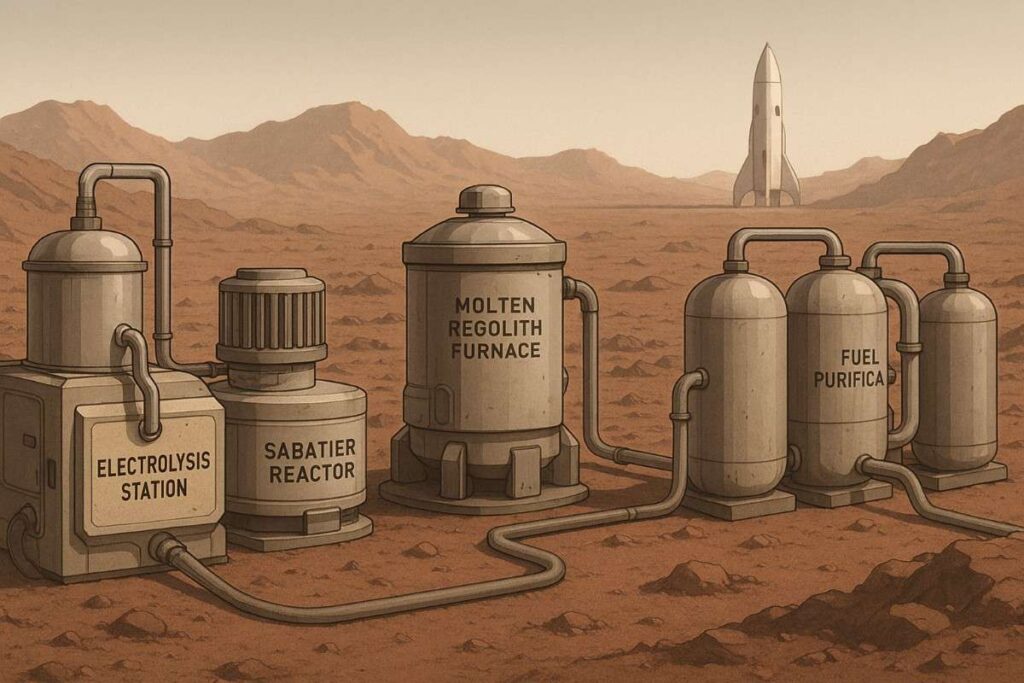
No matter where we are – Moon, Mars, or an asteroid – the process of turning local material into fuel involves deep knowledge of chemistry and engineering. The most common method is electrolysis, which we mentioned already, where electricity splits water into hydrogen and oxygen. This is already done on the International Space Station and will be a big part of future lunar and Martian missions. Power comes from solar energy or nuclear systems.
To turn Martian air into fuel, we use the Sabatier reaction, which combines CO2 and hydrogen to make methane and water. It’s a good way to produce rocket propellant on-site, especially when paired with oxygen from water extraction. Another method is molten regolith electrolysis – heating lunar soil to extreme temperatures to release oxygen. It’s energy-intensive but doesn’t require any water.
Artificial photosynthesis, tested by China, uses catalysts and sunlight to create oxygen and hydrocarbon fuel at room temperature, offering a low-energy alternative for long-term use. And then there’s the thermite reaction, which burns metallic powders with oxidizers to release heat or oxygen. This could be useful for solid fuel rockets or as a backup energy source on the Moon.
In all cases, these methods require a purification process to ensure the fuel is clean and stable for storage and use.
Fuel Stations in Orbit: The Future is Coming
Once we’re producing fuel in space, we’ll need a way to store and use it effectively. That’s where space fuel depots come in. These are essentially gas stations in orbit that can store cryogenic propellants and refuel spacecraft.
NASA and private companies like Orbit Fab are actively working on this. Imagine launching a spacecraft with just enough fuel to reach orbit, then docking at a depot to top up before heading to Mars. It’s more efficient, cheaper, and makes space travel more flexible.
We may also see depots on the Moon, supporting a reusable moon base and even sending propellant to higher orbits. These systems will need to manage solid rocket fuel, liquid hydrogen, and other types, depending on the vehicle.
Looking Ahead
The road to producing fuel beyond Earth is still being paved, but the foundation is strong. Research, missions, and experiments from multiple countries are moving us closer to a future where space infrastructure isn’t just built on Earth, but grown and sustained across the solar system.
Fuel made in space won’t just power rockets. It will support permanent habitats, drive mobility between celestial bodies, and open economic pathways for industries like space mining. The vision of space travel where ships routinely top off at orbital stations or harvest fuel near asteroids is no longer far-fetched – it’s in motion.